THERAKOS® Photopheresis Therapy

What is THERAKOS® Photopheresis?
THERAKOS Photopheresis is an extracorporeal photopheresis (ECP) treatment used by people whose CTCL skin symptoms, like the red, itchy, scaly rash, have not responded to other types of treatments. This therapy is believed to work by harnessing the power of your own immune system, acting from inside your body to treat CTCL skin symptoms.
The exact way that THERAKOS Photopheresis works is not known.
What happens during treatment with THERAKOS® Photopheresis?
US-2500367
Frequently asked questions about THERAKOS® Photopheresis
Remember that your healthcare professional is always the best source of information about your treatment.
What is THERAKOS Photopheresis?
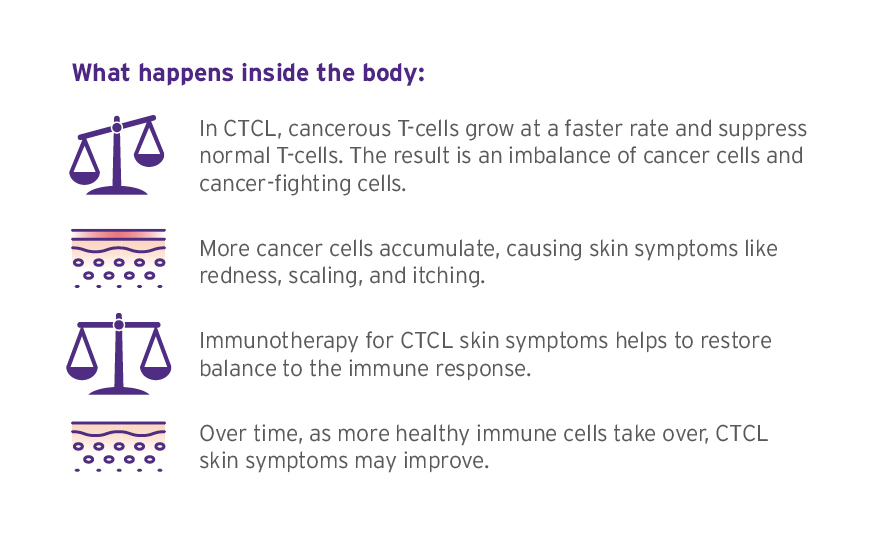
THERAKOS® Photopheresis is a type of treatment called immunotherapy. Immunotherapy is not chemotherapy or radiation. While the exact way that THERAKOS Photopheresis works is not known, based on studies not conducted in humans, it is thought that it may activate an immune response to help fight cancer cells. Over time, as more cancer-fighting cells take over, fewer cancer cells accumulate. This is how CTCL skin symptoms may improve.
Photopheresis, also known as extracorporeal photopheresis (ECP), is a light-activated treatment performed on temporarily separated blood cells outside the body.
The THERAKOS® CELLEX® Photopheresis System delivers ECP using technology that collects, separates, and treats a small number of white blood cells (immune cells) with UVADEX® (methoxsalen) and ultraviolet light while the patient is connected to the instrument.
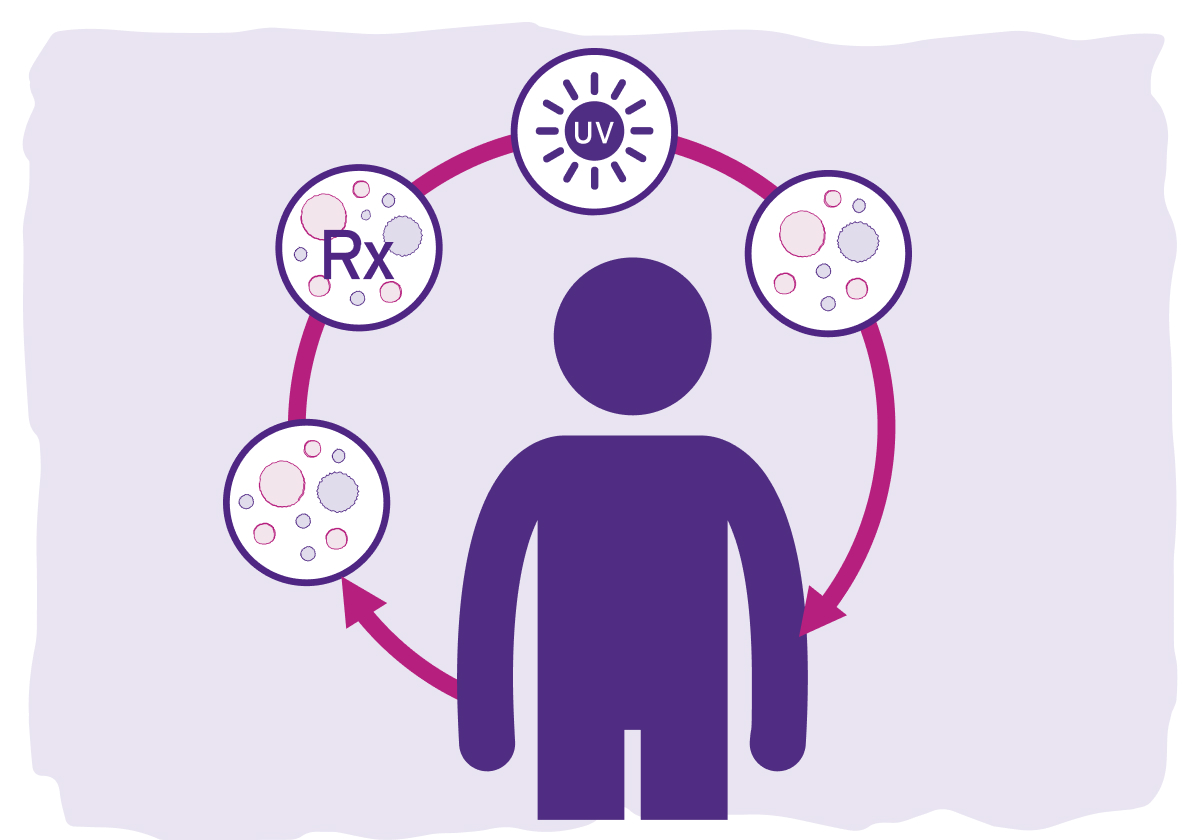
What happens to my blood during the photopheresis process?
During the procedure, a small number of white blood cells are collected, separated from the rest of the blood, treated, and returned back into your body. In fact, only a small number of the white blood cells in your body are needed.
During the THERAKOS Photopheresis process, disposable procedure kits are used only once and thrown out after each treatment. All steps in the treatment process—collecting and separating the blood, treating the white blood cells with medicine and UVA light, and returning the treated cells to the body—happen within one system. Blood is not removed from the THERAKOS Photopheresis closed system.
Be sure to talk with your healthcare professional about the potential side effects of THERAKOS Photopheresis and any other risks. Please see the UVADEX full Prescribing Information for more information on side effects and talk to your doctor.
Why does THERAKOS Photopheresis use my blood?
![]()
CTCL skin symptoms are on the outside of the body, but CTCL cells may also be found in the blood and bone marrow.
![]()
Going inside the body with immunotherapy to target the source of outside symptoms may help CTCL skin symptoms.
Your doctor or nurse will tell you the best way for you to prepare, based on your individual health and medical condition.
Remember to tell your doctor or nurse about any other conditions (such as heart problems, or low blood volume/pressure), as well as any medications you may be taking before starting treatment.
How often would I receive THERAKOS Photopheresis?

*Depending on the instrument and number of intravenous (IV) ports used.
How long will it take to receive each treatment?
The time it takes to get your treatment may vary and will depend on how your healthcare professional decides to deliver it. The CELLEX Photopheresis System can deliver treatment in about 2 hours.*
*Depending on the instrument and number of intravenous (IV) ports used.
When might I see results?
Results may vary from person to person. It is not possible to know if and how well this therapy will work for you. Although you may see results sooner, your doctor may prescribe treatment for a minimum of 6 months. Your doctor will talk with you about how long your therapy should continue. There is no evidence to show that treatment with UVADEX beyond 6 months provided additional benefit.

Do I have to spend the night in the hospital?
Most people with CTCL skin symptoms get photopheresis as an outpatient procedure. They receive the treatment sitting in a lounge chair or lying back in a hospital bed at an infusion center. While getting treatment, you may be able to read, watch TV, email, or nap to pass the time. Once the treatment is done, you are able to go home.
Each person is different. Your healthcare team will watch how you respond to treatment and decide what is best for you. Be sure to tell your healthcare team how you are feeling.

What can I expect?

Where can I find the nearest THERAKOS Photopheresis treatment center?
THERAKOS Photopheresis treatment is given at an infusion center. These centers are most often found at larger hospitals that are part of or work together with a local university. Ask your healthcare team about the nearest treatment center to you.
Treatment centers are independent, third-party facilities not owned or operated by Therakos LLC.
Is THERAKOS Photopheresis FDA approved?
The safety of THERAKOS Photopheresis has been studied in clinical trials. THERAKOS Photopheresis is the only extracorporeal photopheresis (ECP) treatment approved by the US Food and Drug Administration (FDA). It is used to treat the skin symptoms of CTCL in people who have not responded to other types of treatment prescribed by their doctors. It has been used since 1987.
Who should not receive THERAKOS Photopheresis?
Your doctor will decide if THERAKOS Photopheresis is the right treatment for you. People who should not receive treatment include those who:
- Are allergic or have hypersensitivity to methoxsalen, 8-methoxypsoralen, psoralen, or any product similar to related to these products, or any ingredients in UVADEX
- Have a history of a light-sensitive condition or disease
- Have had an eye lens removed; use of UVADEX in this condition can increase the risk of retinal damage
- Have a condition that makes you unable to tolerate loss of blood volume
- Have been diagnosed with a blood-clotting or bleeding problem that can lead to either a blood clot or excessive bleeding
- Have had your spleen removed
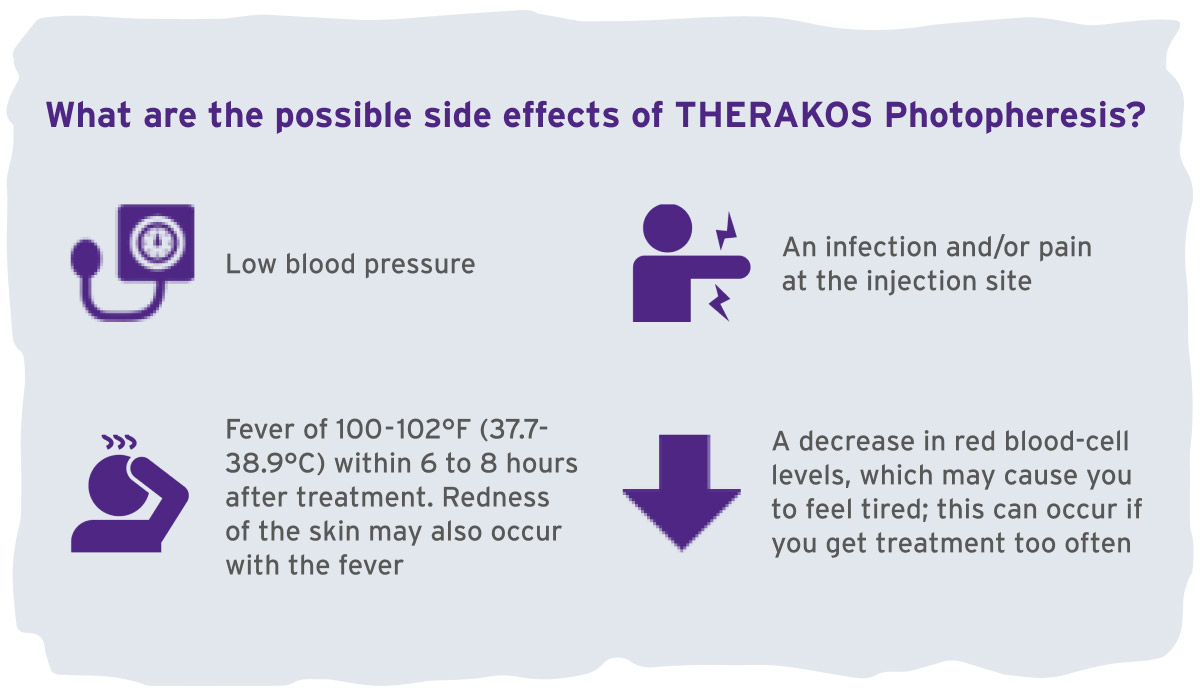
These are not all of the possible side effects of THERAKOS Photopheresis. Please see the UVADEX full Prescribing Information for more details and talk to your doctor. Tell your doctor about any side effects that bother you or that do not go away. Call your doctor for medical advice about side effects. You may report side effects to the FDA. Call 1-800-FDA-1088 or visit www.fda.gov/medwatch. You may also report side effects by calling Therakos at 1-877-566-9466.
Why do I have to be careful about being in the sun and wearing sunglasses after treatment?
THERAKOS Photopheresis treatment may make you more sensitive to the sun. That is why you should avoid sunlight for the 24 hours after treatment. This includes direct and indirect sunlight, either outdoors or even through a window.
This is not all the information you will need to know about sun sensitivity during THERAKOS Photopheresis treatment. Please see the UVADEX full Prescribing Information for more details. Be sure to talk with your doctor about them and to learn what you need to do during treatment.
Talking with your doctor about THERAKOS® Photopheresis
It is important that you take an active role in your healthcare. If you and your doctor are thinking about THERAKOS Photopheresis, be sure you have an open, candid conversation about treatment.
It may help to bring along a friend or loved one who can listen, ask questions you may not think of, and help you remember your doctor’s answers.
Below are some questions you may want to ask:
What causes CTCL skin symptoms?
Is there a cure for CTCL skin symptoms?
Am I a candidate for photopheresis?
How might photopheresis help my CTCL skin symptoms?
What are some of the possible side effects of THERAKOS Photopheresis?
What is involved in getting photopheresis?
Is treatment painful?
What would the treatment schedule be?
How long would I receive photopheresis?
What would I need to do to prepare for photopheresis treatment?








